Abstract
Introduction: Outcomes are dismal for patients (pts) with relapsed/refractory (R/R) AML. Innate and adaptive immune aberrations exist in AML pts leading to immune escape. We have previously shown that there is an increased expression of multiple co-inhibitory molecules including programmed death-1 (PD-1) in the peripheral blood of AML pts at diagnosis, and these levels persist in pts with refractory disease (Knaus H, et al. Blood [Abstract] 2015). We hypothesized that administration of pembrolizumab after high dose cytarabine (HiDAC) salvage chemotherapy would stimulate a T-cell mediated anti-leukemic immune response leading to improved efficacy in R/R AML.
Methods: A multicenter phase II study of age-adjusted HiDAC (<60 years: 2 gm/m2 IV Q12hours days 1-5; >=60 years: 1.5 gm/m2 IV Q12hours days 1-5) followed by pembrolizumab 200 mg IV on day 14 is being conducted in R/R AML pts 18-70 years. The primary endpoint of this study is the rate of complete remission (CR)/CR with incomplete recovery (CRi) after HiDAC followed by pembrolizumab. The study design is a Simon's like two-stage design (n=37 pts) with relaxed stopping for futility. Relaxed stopping refers to overall response rate (CR/CRi+partial remission [PR]) when stopping rule for futility is applied in the first stage. The null hypothesis that the CR/CRi rate for HiDAC followed by pembrolizumab is 20% will be tested against a one-sided alternative hypothesis yielding a type 1 error rate = 5% and power = 84% when the CR/CRi rate of HiDAC followed by pembrolizumab = 40%. Continuous monitoring for toxicity will be performed to assess for unacceptable toxicity. Overall responders will receive maintenance phase pembrolizumab 200 mg IV Q3weeks for up to 2 years until relapse/progression. Pts can proceed to allogeneic stem cell transplant (SCT) before or after maintenance phase (SCT must be >=30 days from last dose of pembrolizumab). Correlative studies include serial peripheral blood and bone marrow flow cytometry, mRNA-sequencing, and T cell receptor (TCR) clonality studies to determine predictive biomarkers of response.
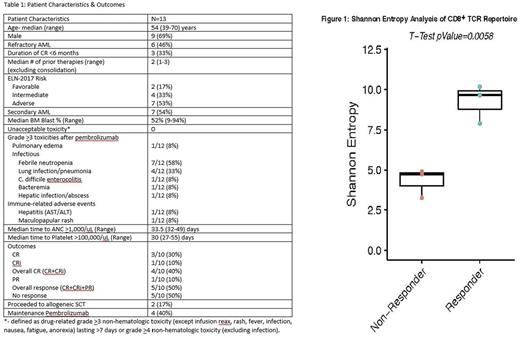
Results: 13 pts enrolled on this study between August, 2016-July, 2017 (Table 1). There have been 0 unacceptable toxicities to date. Immune-related adverse events have included grade 2 hyperbilirubinemia (n=1), grade 3 hepatic enzymes (n=1), both resolved promptly after systemic corticosteroids, as well as self-resolving grade 3 rash (n=1). Additionally, 1 pt developed grade 3 pulmonary edema without evidence of pneumonitis that resolved spontaneously without steroids. Of the 10 evaluable pts (as of July 1, 2017), overall response rate = 50% (CR/CRi: 4/10= 40%; PR = 1/10=10%), meeting the threshold to proceed to stage 2. Two pts have proceeded to a myeloablative allogeneic SCT in CR (one of whom received 2 cycles of maintenance pembrolizumab) and remain in CR. Post-SCT, both pts developed steroid-responsive acute GVHD of skin, one pt developed a transient increase in hepatic enzymes that was responsive to steroids, and one pt developed moderate chronic GVHD. Four pts have received maintenance pembrolizumab: 2 pts relapsed (median duration of CR = 3.9 months; range: 2-5.7 months), one pt proceeded to SCT after 2 cycles, and one pt initially achieved a PR and remains with stable disease after 11 cycles of maintenance pembrolizumab. No pts have died in remission. 30-day and 60-day mortality = 0% and 10%, respectively. Preliminary findings from the first 6 pts (3 responders vs. 3 non-responders) revealed increased diversity of TCR Vβ repertoire from pre-treatment peripheral blood CD8+ T-cells in responders when compared with non-responders using Shannon entropy analysis (p=0.0058; Figure 1).
Conclusions: Early results of a multicenter phase II study demonstrate the feasibility of adding immune checkpoint blockade with pembrolizumab after HiDAC chemotherapy in R/R AML. An overall response rate of 50% is encouraging in a high-risk R/R AML cohort though ongoing findings are imperative to evaluate the efficacy and future role of pembrolizumab in AML. Interestingly, in a small cohort of patients, broadening of the immune repertoire, perhaps by increasing T cell responses beyond endogenous viral responses, was associated with response to PD-1 blockade. Future directions include a comprehensive immune biomarker discovery approach to determine predictors of response to immune checkpoint blockade in AML.
Zeidner: Tolero Pharmaceuticals: Honoraria; Agios: Honoraria; Merck: Research Funding; Takeda: Research Funding; Tolero Pharmaceuticals: Research Funding; Celgene: Honoraria. Vincent: Merck: Research Funding; Pharmacyclics: Research Funding; Nanostring: Membership on an entity's Board of Directors or advisory committees. Foster: Shire: Honoraria; Incyte: Honoraria; Macrogenics: Research Funding; Celgene: Research Funding; Amgen: Honoraria; Pfizer: Research Funding; Celator: Research Funding. Coombs: Pharmacyclics: Honoraria; H3 Biomedicine: Honoraria. Gojo: Merck: Research Funding; Amgen: Research Funding; Amgen: Honoraria; Novartis: Research Funding. Serody: Merck: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal